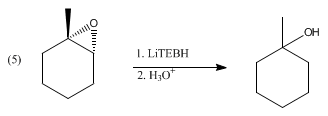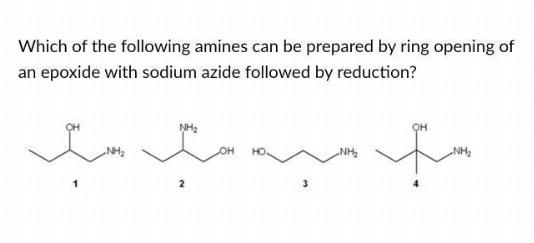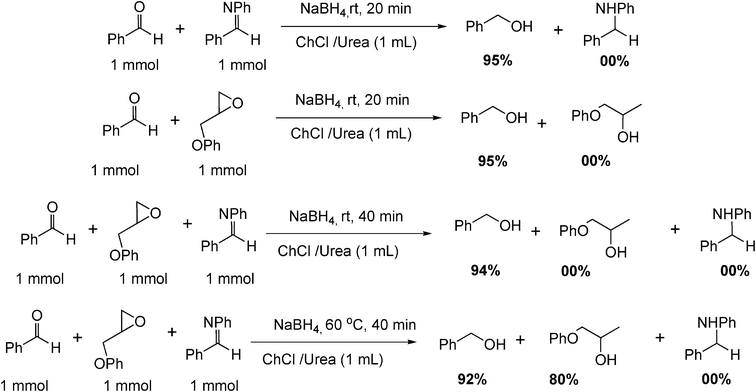
Natural deep eutectic salt promoted regioselective reduction of epoxides and carbonyl compounds - RSC Advances (RSC Publishing) DOI:10.1039/C2RA01280D

Catalytic reductive ring opening of epoxides enabled by zirconocene and photoredox catalysis - ScienceDirect

Synthesis of α-Alkylated Ketones via Selective Epoxide Opening/Alkylation Reactions with Primary Alcohols | Organic Letters
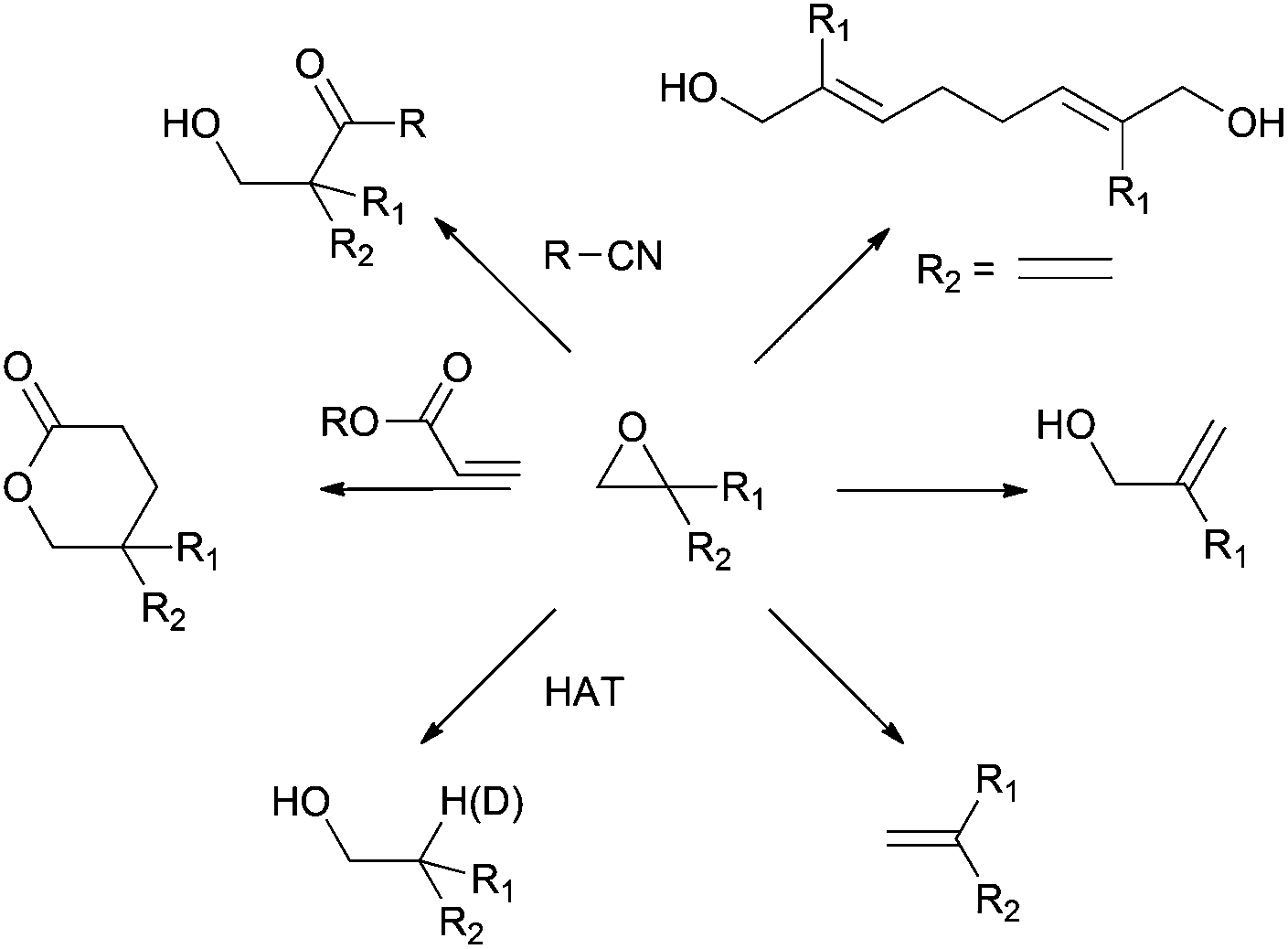
Recent applications of Cp 2 TiCl in natural product synthesis - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C3QO00024A

Catalytic Hydrogenation of Epoxides to Alcohols - Thiyagarajan - 2022 - Chemistry – An Asian Journal - Wiley Online Library

A New and Efficient Epoxide Ring Opening via Poor Nucleophiles: Indole, p-Nitroaniline, Borane and O-Trimethylsilylhydroxylamine in Lithium Perchlorate

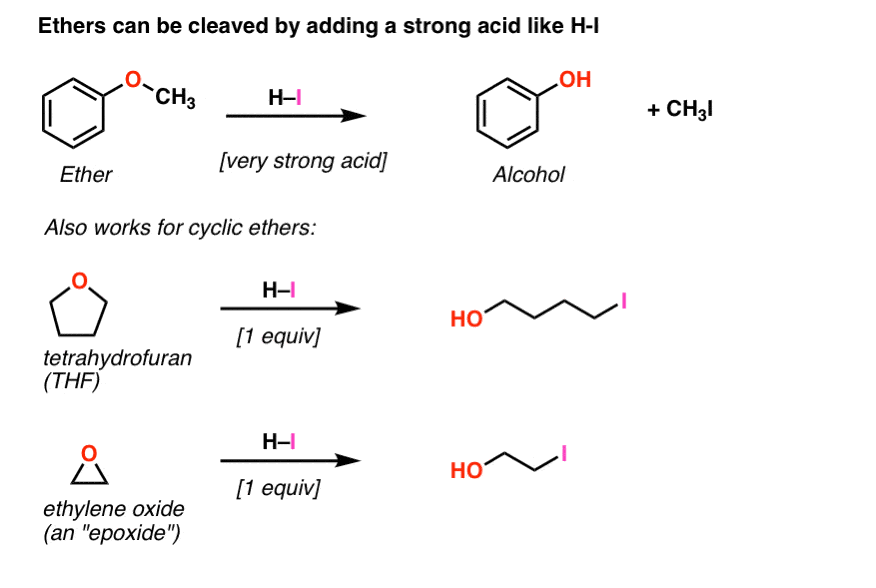
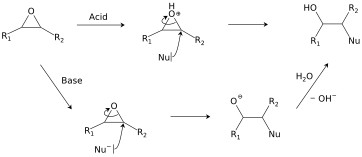
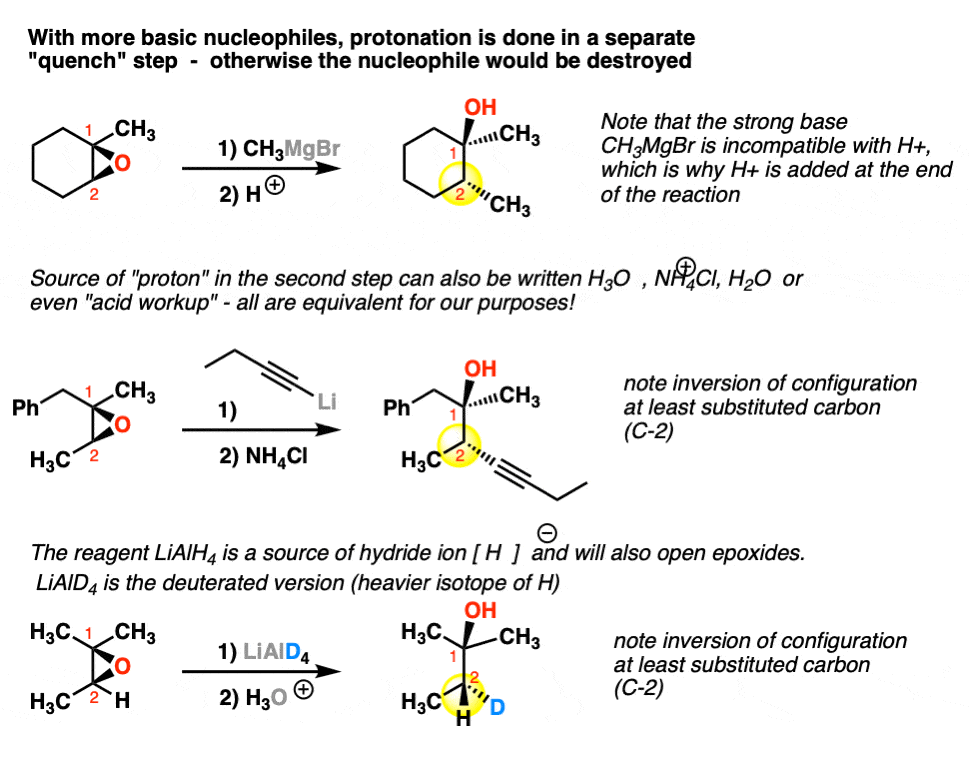
This explanation says that there is an inversion in stereochemistry when the reducing agent reduces the epoxide, but the stereochemistry after reduction stays the same. Can anyone explain? : r/OrganicChemistry

Iron-catalysed regioselective hydrogenation of terminal epoxides to alcohols under mild conditions | Nature Catalysis